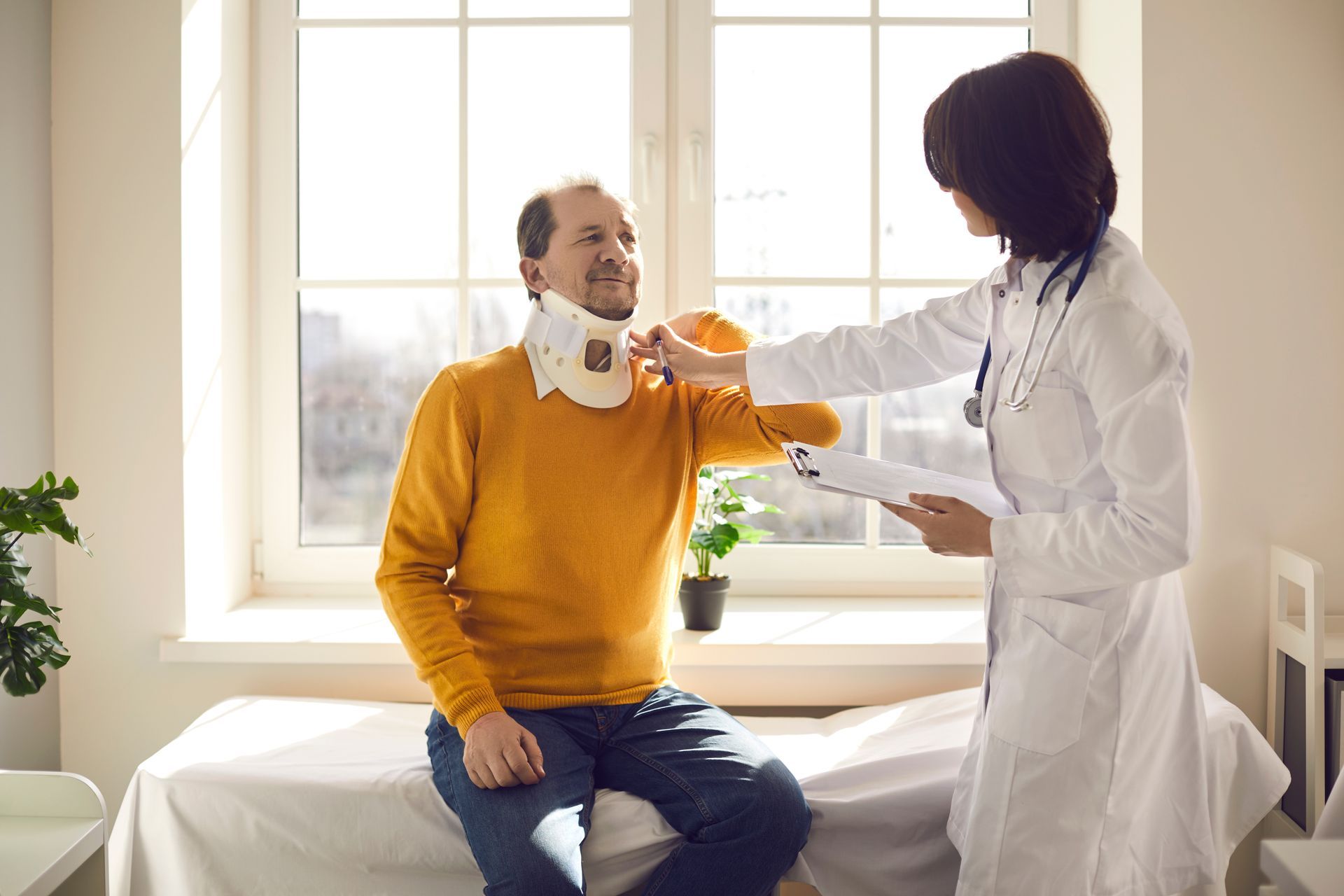
Most medications undergo rigorous testing before they’re released on the market here in the United States. Government oversight over these prescription drugs doesn’t end once they’re made available to the public though. The U.S. Food and Drug Administration (FDA) continues to monitor medications for potential problems. They keep a log of any reports that are made to them. The FDA may recall a drug if they fear that it may pose a safety hazard.
Both prescription and over-the-counter drugs can be recalled. Recalls may happen for various reasons.
One of the more common reasons why drug recalls occur is because it poses some type of health hazard. While many of these medications are tested before being made widely available, the control groups that they’re given to are fairly small. Many of these small-scale studies don’t reveal the full set of risks associated with a drug. It’s only once it’s prescribed to a larger sample of patients that manufacturers and regulators understand more about the adverse side effects associated with patients taking a certain medication.
Another common reason why drugs get recalled is due to contamination. Medications can be contaminated by either nonharmful or harmful substances during the manufacturing or distribution process. This could expose patients to other illnesses or make their medication not work as intended.
It’s not unheard of for medications to be recalled due to improper labeling or packaging either. A pill may be labeled with the wrong markings. The dosage instructions written on the packaging may be confusing or inaccurate. The package that the medication comes may not be child-resistant and puts them at a risk for injury or death. Different over-the-counter and prescription drugs have been recalled for these exact reasons in recent years.
Many drug manufacturers continue to perform clinical research and testing on their medications after introducing them to the market. This often results in manufacturers voluntarily recalling their drugs. Many recalls happen after the FDA receives alarming reports about side effects that patients have suffered from taking medication though.